Bayesian Methods for Multilevel Modeling of Neurons
Contents
Bayesian Methods for Multilevel Modeling of Neurons¶
This is a slight reworking of the radon example from pymc3 https://docs.pymc.io/notebooks/multilevel_modeling.html
Why this notebook? It’s a common departure point to consider multilevel models in neuroscience.
Implementations in:
tensorflow https://www.tensorflow.org/probability/examples/Multilevel_Modeling_Primer
pymc3 https://docs.pymc.io/notebooks/multilevel_modeling.html
stan https://mc-stan.org/users/documentation/case-studies/radon.html
pyro https://github.com/pyro-ppl/pyro-models/blob/master/pyro_models/arm/radon.py
numpyro fibrosis dataset http://num.pyro.ai/en/stable/tutorials/bayesian_hierarchical_linear_regression.html
Hierarchical or multilevel modeling is a generalization of regression modeling. Multilevel models are regression models in which the constituent model parameters are given probability models. This implies that model parameters are allowed to vary by group. Observational units are often naturally clustered. Clustering induces dependence between observations, despite random sampling of clusters and random sampling within clusters.
A hierarchical model is a particular multilevel model where parameters are nested within one another. Some multilevel structures are not hierarchical – e.g. “country” and “year” are not nested, but may represent separate, but overlapping, clusters of parameters. We will motivate this topic using a neuroscience example.
Example: firing rate (modified Gelman and Hill 2006)
Firing rate is a measure of neuronal activity. It varies greatly from neuron to neuron.
The EPA did a study of radon levels in firing rate in 80,000 households (more on this study and modeling here https://pymc3-testing.readthedocs.io/en/rtd-docs/notebooks/multilevel_modeling.html). We are going to call homes “neurons”, counties “mice”, and radon levels “firing rate.” The firing rate is going to vary depending on “no_stim” (originally floor condition) or “stim” (originally basement condition) that in our case denote, say, optogenetic stimulation of prefrontal cortex. There are two important predictors:
measurement in stim or first no_stim (firingrate higher in stims)
mouse firing rate level (positive correlation with firingrate levels by mouse)
The hierarchy in this example is neurons within mouse.
Note that we are not considering the variability that comes from house identity, which would mean neuron identity in our neuroscience language. This means that each neuron/house has one measurement. The estimation of firing rate of a neuron is thus a separate topic.
Data organization¶
First, we import the data from a local file, and extract data.
# HIDE CODE
import arviz as az
import matplotlib.pyplot as plt
import numpy as np
import pandas as pd
import pymc3 as pm
import warnings
from theano import tensor as tt
warnings.simplefilter(action="ignore", category=FutureWarning)
RANDOM_SEED = 8924
np.random.seed(286)
# HIDE CODE
az.style.use("arviz-darkgrid")
# Import firingrate data
srrs2 = pd.read_csv(pm.get_data('srrs2.dat'))
srrs2.columns = srrs2.columns.map(str.strip)
# Select a subset of "mice" from Minnesota
srrs_mn = srrs2[srrs2.state=='MN'].copy()
Next, obtain the mouse-level predictor, firing rate, by combining two variables.
# HIDE CODE
srrs_mn['fips'] = srrs_mn.stfips*1000 + srrs_mn.cntyfips
cty = pd.read_csv(pm.get_data('cty.dat'))
cty_mn = cty[cty.st=='MN'].copy()
cty_mn[ 'fips'] = 1000*cty_mn.stfips + cty_mn.ctfips
# HIDE CODE
print(srrs_mn.columns,srrs_mn.shape)
print(cty_mn.columns,cty_mn.shape)
Index(['idnum', 'state', 'state2', 'stfips', 'zip', 'region', 'typebldg',
'floor', 'room', 'basement', 'windoor', 'rep', 'stratum', 'wave',
'starttm', 'stoptm', 'startdt', 'stopdt', 'activity', 'pcterr', 'adjwt',
'dupflag', 'zipflag', 'cntyfips', 'county', 'fips'],
dtype='object') (919, 26)
Index(['stfips', 'ctfips', 'st', 'cty', 'lon', 'lat', 'Uppm', 'fips'], dtype='object') (89, 8)
Use the merge method to combine neuron- and mouse-level information in a single DataFrame.
# HIDE CODE
srrs_mn = srrs_mn.merge(cty_mn[['fips', 'Uppm']], on='fips')
srrs_mn = srrs_mn.drop_duplicates(subset='idnum')
u = np.log(srrs_mn.Uppm).unique()
n = len(srrs_mn)
# HIDE CODE
srrs_mn['Uppm'].unique().shape,srrs_mn['activity'].unique().shape
#plt.scatter(srrs_mn['Uppm'],srrs_mn['activity'])
((85,), (156,))
# HIDE CODE
# Rename environmental variables to represent
# what we think of as a neuroscience example
srrs_mn.rename({'floor':'no_stim','basement':'stim','county':'mouse',
'activity':'firingrate'},axis=1,inplace=True)
# HIDE CODE
srrs_mn.head()
| idnum | state | state2 | stfips | zip | region | typebldg | no_stim | room | stim | ... | stopdt | firingrate | pcterr | adjwt | dupflag | zipflag | cntyfips | mouse | fips | Uppm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5081 | MN | MN | 27 | 55735 | 5 | 1 | 1 | 3 | N | ... | 12288 | 2.2 | 9.7 | 1146.499190 | 1 | 0 | 1 | AITKIN | 27001 | 0.502054 |
| 1 | 5082 | MN | MN | 27 | 55748 | 5 | 1 | 0 | 4 | Y | ... | 12088 | 2.2 | 14.5 | 471.366223 | 0 | 0 | 1 | AITKIN | 27001 | 0.502054 |
| 2 | 5083 | MN | MN | 27 | 55748 | 5 | 1 | 0 | 4 | Y | ... | 21188 | 2.9 | 9.6 | 433.316718 | 0 | 0 | 1 | AITKIN | 27001 | 0.502054 |
| 3 | 5084 | MN | MN | 27 | 56469 | 5 | 1 | 0 | 4 | Y | ... | 123187 | 1.0 | 24.3 | 461.623670 | 0 | 0 | 1 | AITKIN | 27001 | 0.502054 |
| 4 | 5085 | MN | MN | 27 | 55011 | 3 | 1 | 0 | 4 | Y | ... | 13088 | 3.1 | 13.8 | 433.316718 | 0 | 0 | 3 | ANOKA | 27003 | 0.428565 |
5 rows × 27 columns
We also need a lookup table (dict) for each unique mouse, for indexing.
# HIDE CODE
srrs_mn.mouse = srrs_mn.mouse.map(str.strip)
mn_mice = srrs_mn.mouse.unique()
n_mice = len(mn_mice)
mouse_lookup = dict(zip(mn_mice, range(n_mice)))
Finally, create local copies of variables.
# HIDE CODE
mouse = srrs_mn['mouse_code'] = srrs_mn.mouse.replace(mouse_lookup).values
firingrate = srrs_mn.firingrate
srrs_mn['log_firingrate'] = log_firingrate = np.log(firingrate + 0.1).values
no_stim = srrs_mn.no_stim.values
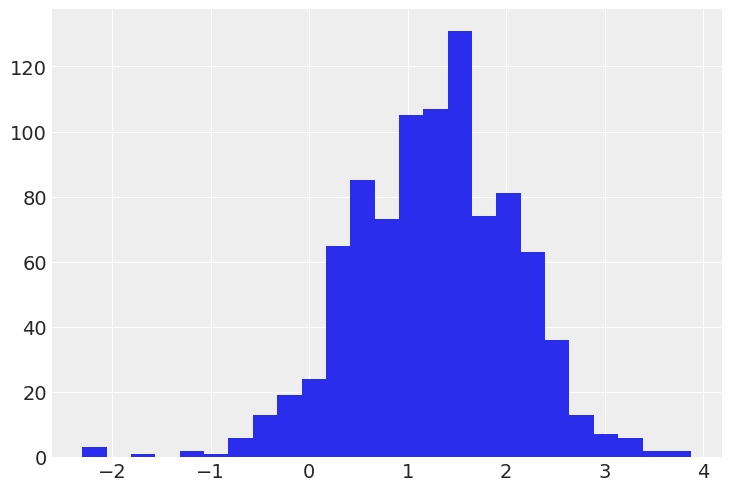
Distribution of firing rate levels (log scale):
# HIDE CODE
srrs_mn.log_firingrate.hist(bins=25);
Conventional approaches¶
The two conventional alternatives to modeling firing rate exposure represent the two extremes of the bias-variance tradeoff:
Complete pooling:
Treat all mice the same, and estimate a single firing rate level.
No pooling:
Model firing rate in each mouse independently.
where \(j = 1,\ldots,85\)
The errors \(\epsilon_i\) may represent measurement error, temporal within-neuron variation, or variation among neurons.
We’ll start by estimating the slope and intercept for the complete pooling model. You’ll notice that we used an index variable instead of an indicator variable in the linear model below. There are two main reasons. One, this generalizes well to more-than-two-category cases. Two, this approach correctly considers that neither category has more prior uncertainty than the other. On the contrary, the indicator variable approach necessarily assumes that one of the categories has more uncertainty than the other: here, the cases when no_stim=1 would take into account 2 priors (\(\alpha + \beta\)), whereas cases when no_stim=0 would have only one prior (\(\alpha\)). But a priori we aren’t more unsure about no_stim measurements than about stim measurements, so it makes sense to give them the same prior uncertainty.
Now for the model:
# HIDE CODE
with pm.Model() as pooled_model:
a = pm.Normal('a', 0., sigma=10., shape=2)
theta = a[no_stim]
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(pooled_model)
Before running the model let’s do some prior predictive checks. Indeed, having sensible priors is not only a way to incorporate scientific knowledge into the model, it can also help and make the MCMC machinery faster – here we are dealing with a simple linear regression, so no link function comes and distorts the outcome space; but one day this will happen to you and you’ll need to think hard about your priors to help your MCMC sampler. So, better to train ourselves when it’s quite easy than having to learn when it’s very hard… There is a really neat function to do that in PyMC3:
# HIDE CODE
with pooled_model:
prior_checks = pm.sample_prior_predictive(random_seed=RANDOM_SEED)
plt.plot(
[0, 1],
[prior_checks["a"][:, 0], prior_checks["a"][:, 1]],
"ok",
alpha=0.2)
plt.xlabel("no_stim measurement (binary)")
plt.xticks([0,1], ["stim", "no_stim"])
plt.ylabel("Mean log firingrate level");
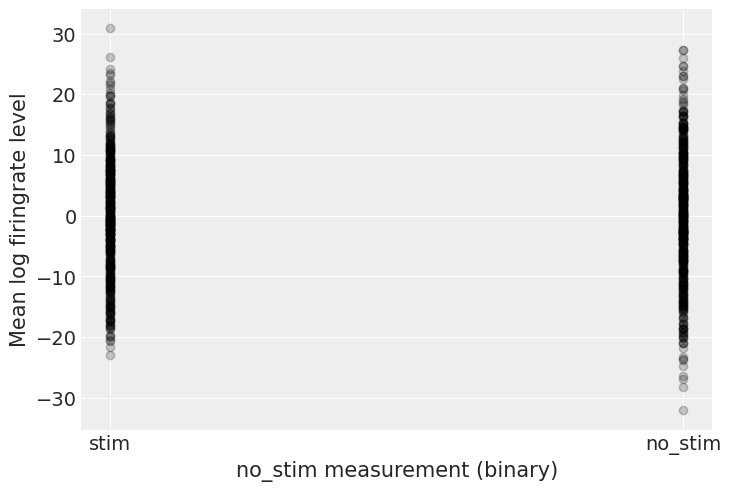
I’m no expert in firingrate levels, but, before seing the data, these priors seem to allow for quite a wide range of the mean log firingrate level. But don’t worry, we can always change these priors if sampling gives us hints that they might not be appropriate – after all, priors are assumptions, not oaths; and as most assumptions, they can be tested.
However, we can already think of an improvement. Do you see it? Remember what we said at the beginning: firingrate levels tend to be higher in stims, so we could incorporate this prior scientific knowledge into our model by giving \(a_{stim}\) a higher mean than \(a_{no_stim}\). Here, there are so much data that the prior should be washed out anyway, but we should keep this fact in mind – for future cases or if sampling proves more difficult than expected…
Speaking of sampling, let’s fire up the Bayesian machinery!
# HIDE CODE
with pooled_model:
pooled_trace = pm.fit()
Finished [100%]: Average Loss = 1,149.9
# HIDE CODE
with pooled_model:
start = pm.find_MAP()
pooled_trace = pm.sample(1000, pm.NUTS(scaling=start), start=start, random_seed=RANDOM_SEED)
az.summary(pooled_trace, round_to=2)
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, a]
Sampling 4 chains for 1_000 tune and 1_000 draw iterations (4_000 + 4_000 draws total) took 2 seconds.
| mean | sd | hdi_3% | hdi_97% | mcse_mean | mcse_sd | ess_bulk | ess_tail | r_hat | |
|---|---|---|---|---|---|---|---|---|---|
| a[0] | 1.36 | 0.03 | 1.31 | 1.42 | 0.0 | 0.0 | 5266.08 | 2963.49 | 1.0 |
| a[1] | 0.78 | 0.06 | 0.66 | 0.90 | 0.0 | 0.0 | 5856.73 | 3357.46 | 1.0 |
| sigma | 0.79 | 0.02 | 0.76 | 0.83 | 0.0 | 0.0 | 5335.04 | 2973.88 | 1.0 |
# HIDE CODE
with pooled_model:
pooled_trace = pm.sample(1000, tune=2000, random_seed=RANDOM_SEED)
az.summary(pooled_trace, round_to=2)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, a]
Sampling 4 chains for 2_000 tune and 1_000 draw iterations (8_000 + 4_000 draws total) took 3 seconds.
| mean | sd | hdi_3% | hdi_97% | mcse_mean | mcse_sd | ess_bulk | ess_tail | r_hat | |
|---|---|---|---|---|---|---|---|---|---|
| a[0] | 1.36 | 0.03 | 1.31 | 1.42 | 0.0 | 0.0 | 4702.01 | 2808.27 | 1.0 |
| a[1] | 0.77 | 0.06 | 0.65 | 0.90 | 0.0 | 0.0 | 4962.17 | 2066.08 | 1.0 |
| sigma | 0.79 | 0.02 | 0.76 | 0.83 | 0.0 | 0.0 | 4176.84 | 2823.50 | 1.0 |
No divergences and a sampling that only took seconds – this is the Flash of samplers! Here the chains look very good (good R hat, good effective sample size, small sd), but remember to check your chains after sampling – az.traceplot is usually a good start.
Let’s see what it means on the outcome space: did the model pick-up the negative relationship between no_stim measurements and log firingrate levels? What’s the uncertainty around its estimates? To estimate the uncertainty around the neuron firingrate levels (not the average level, but measurements that would be likely in neurons), we need to sample the likelihood y from the model. In another words, we need to do posterior predictive checks:
# HIDE CODE
with pooled_model:
ppc = pm.sample_posterior_predictive(pooled_trace, random_seed=RANDOM_SEED)["y"]
a_stim, a_no_stim = pooled_trace["a"].mean(axis=0)
firingrate_stim, firingrate_no_stim = ppc[:, 1], ppc[:, 0] # we know that no_stim=0/1 at these columns
We can then use these samples in our plot:
# HIDE CODE
plt.scatter(no_stim, log_firingrate, label="Observations", alpha=0.4)
az.plot_hdi(
[0, 1],
np.asarray([firingrate_stim, firingrate_no_stim]).T,
fill_kwargs={"alpha": 0.2, "label": "Exp. distrib. of firingrate levels"}
)
az.plot_hdi(
[0, 1],
pooled_trace["a"],
fill_kwargs={"alpha": 0.5, "label": "Exp. mean HPD"}
)
plt.plot([0, 1], [a_stim, a_no_stim], label="Exp. mean")
plt.xticks([0,1], ["stim", "no_stim"])
plt.xlabel("no_stim measurement (binary)")
plt.ylabel("Log firingrate level")
plt.legend(ncol=2, fontsize=9, frameon=True);
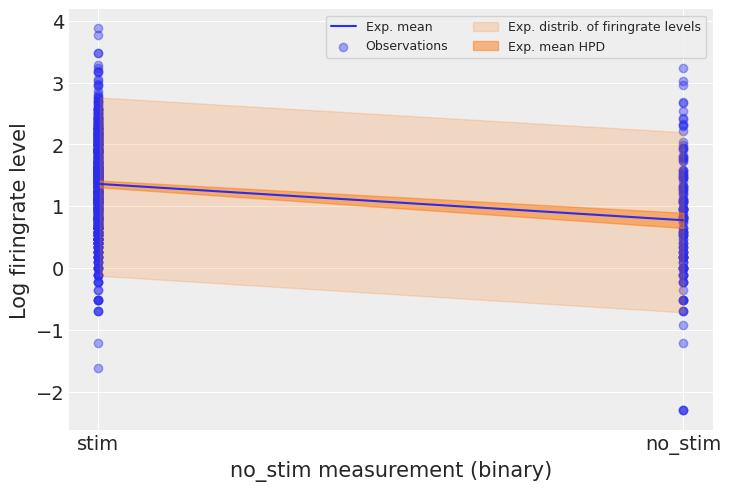
The 94% interval of the expected value is very narrow, and even narrower for stim measurements, meaning that the model is slightly more confident about these observations. The sampling distribution of individual firingrate levels is much wider. We can infer that no_stim level does account for some of the variation in firingrate levels. We can see however that the model underestimates the dispersion in firingrate levels across neurons – lots of them lie outside the light orange prediction envelope. So this model is a good start but we can’t stop there.
Let’s compare it to the unpooled model, where we estimate the firingrate level for each mouse:
# HIDE CODE
n_mice,no_stim.shape,log_firingrate.shape
(85, (919,), (919,))
# HIDE CODE
# updated version:
with pm.Model() as unpooled_model:
a = pm.Normal('a', 0., sigma=10., shape=(n_mice, 2))
theta = a[mouse, no_stim]
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(unpooled_model)
# HIDE CODE
with unpooled_model:
unpooled_trace = pm.sample(1000, tune=2000, random_seed=RANDOM_SEED)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, a]
Sampling 4 chains for 2_000 tune and 1_000 draw iterations (8_000 + 4_000 draws total) took 8 seconds.
Sampling went fine again. Let’s look at the expected values for both stim (dimension 0) and no_stim (dimension 1) in each mouse:
# HIDE CODE
az.plot_forest(unpooled_trace, var_names=['a'], figsize=(6, 32), r_hat=True, combined=True);
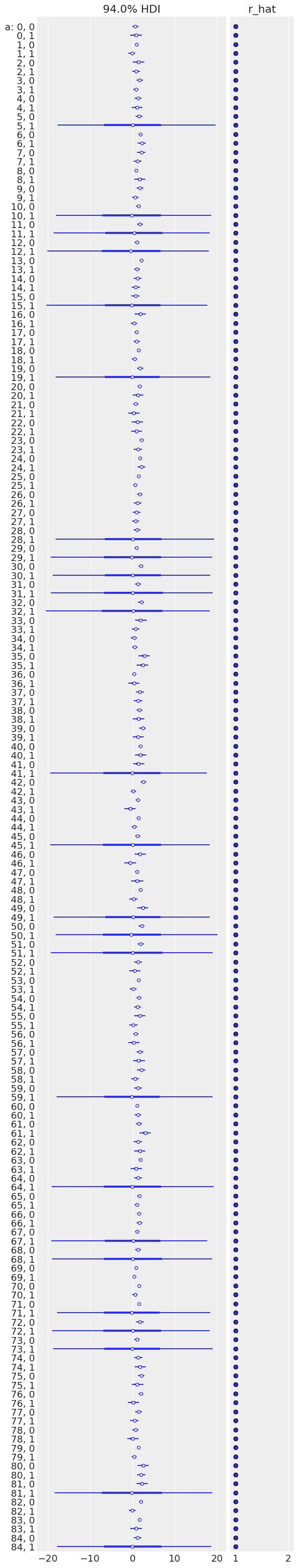
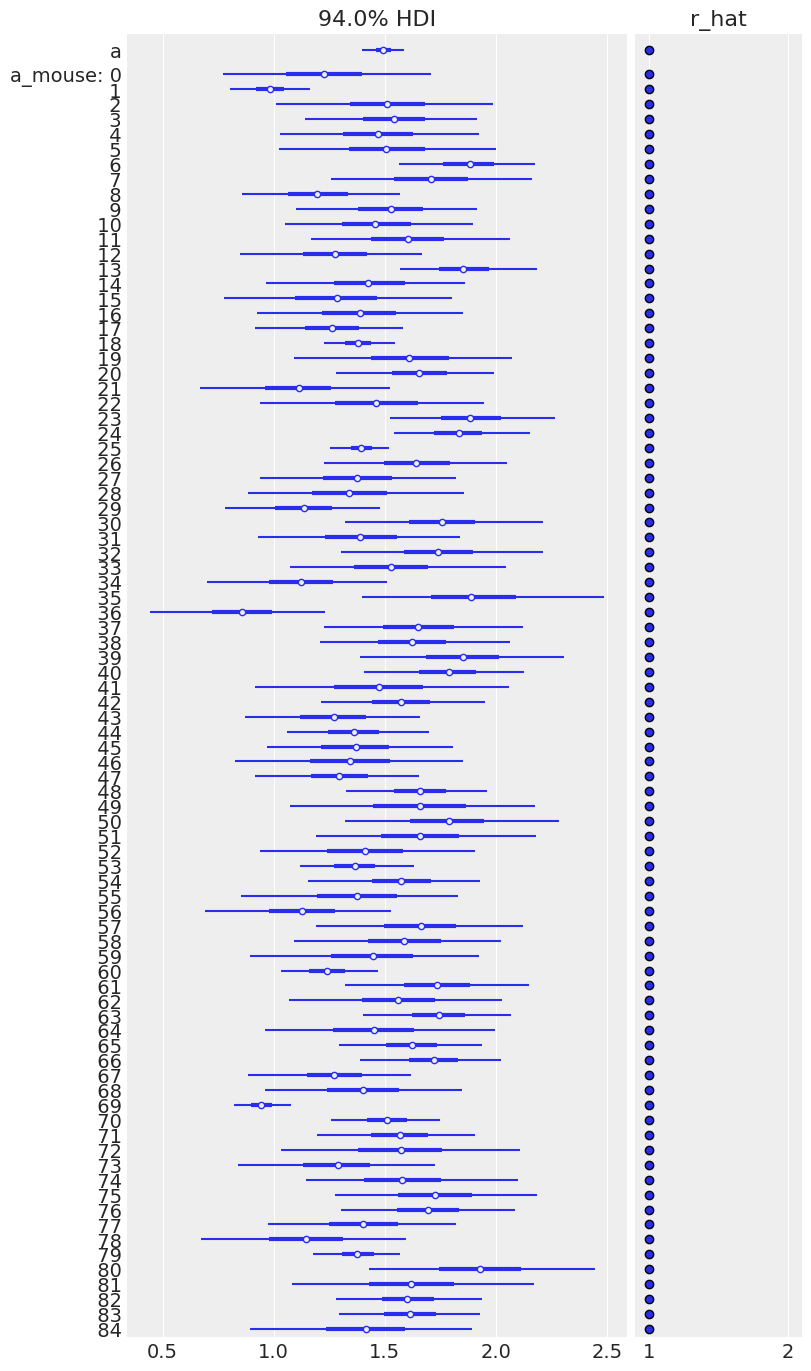
Sampling was good for all mice, but you can see that some are more uncertain than others, and all of these uncertain estimates are for no_stim measurements. This probably comes from the fact that some mice just have a handful of no_stim measurements, so the model is pretty uncertain about them.
To identify mice with high firing rate levels, we can plot the ordered mean estimates, as well as their 94% HPD:
# HIDE CODE
a_stim_unpooled, a_no_stim_unpooled = unpooled_trace["a"][:, :, 0], unpooled_trace["a"][:, :, 1]
unpooled_stim = pd.DataFrame.from_dict(
{
"stim": a_stim_unpooled.mean(0),
"low": az.hdi(a_stim_unpooled)[:, 0],
"high": az.hdi(a_stim_unpooled)[:, 1]
},
orient="index",
columns=mn_mice
).T.sort_values(by="stim")
unpooled_no_stim = pd.DataFrame.from_dict(
{
"no_stim": a_no_stim_unpooled.mean(0),
"low": az.hdi(a_no_stim_unpooled)[:, 0],
"high": az.hdi(a_no_stim_unpooled)[:, 1]
},
orient="index",
columns=mn_mice
).T.sort_values(by="no_stim")
# HIDE CODE
fig, axes = plt.subplots(1, 2, figsize=(12, 5))
for ax, estimates, level in zip(axes, [unpooled_stim, unpooled_no_stim], ["stim", "no_stim"]):
for i, l, h in zip(range(n_mice), estimates.low.values, estimates.high.values):
ax.plot([i, i], [l, h], alpha=0.6, c='orange')
ax.scatter(range(n_mice), estimates[level], alpha=0.8)
ax.set(title=f"{level.title()} estimates", xlabel="Ordered mouse", xlim=(-1, 86), ylabel="firingrate estimate", ylim=(-2, 4.5))
plt.tight_layout();
<ipython-input-24-8917f1fd6ec1>:9: UserWarning: This figure was using constrained_layout, but that is incompatible with subplots_adjust and/or tight_layout; disabling constrained_layout.
plt.tight_layout();
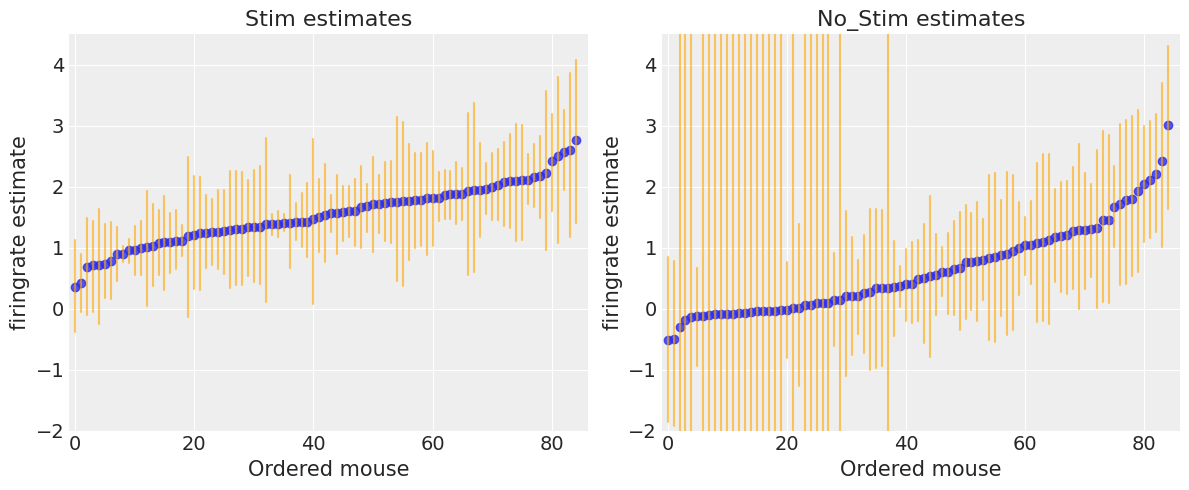
There seems to be more dispersion in firingrate levels for no_stim measurements than for stim ones. Moreover, as we saw in the forest plot, no_stim estimates are globally more uncertain, especially in some mice. We speculated that this is due to smaller sample sizes in the data, but let’s verify it!
# HIDE CODE
n_no_stim_meas = srrs_mn.groupby("mouse").sum().no_stim
uncertainty = (unpooled_no_stim.high - unpooled_no_stim.low).sort_index() # sort index to match counties alphabetically
plt.plot(n_no_stim_meas, uncertainty, 'o', alpha=.4)
plt.xlabel("Nbr no_stim measurements in mouse")
plt.ylabel("Estimates' uncertainty");
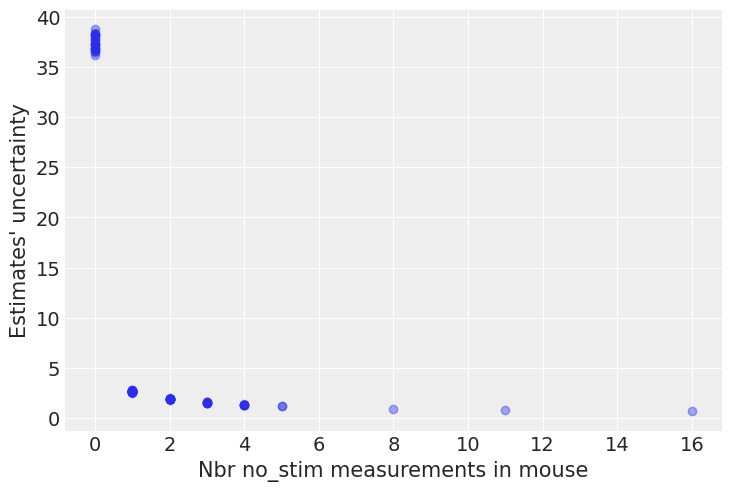
Bingo! This makes sense: it’s very hard to estimate no_stim firingrate levels in mice where there are no no_stim measurements, and the model is telling us that by being very uncertain in its estimates for those mice. This is a classic issue with no-pooling models: when you estimate clusters independently from each other, what do you with small-sample-size mice?
Another way to see this phenomenon is to visually compare the pooled and unpooled estimates for a subset of mice representing a range of sample sizes:
# HIDE CODE
# These mice were named as counties in Minnesota, how curious
SAMPLE_mice = ('LAC QUI PARLE', 'AITKIN', 'KOOCHICHING',
'DOUGLAS', 'CLAY', 'STEARNS', 'RAMSEY', 'ST LOUIS')
fig, axes = plt.subplots(2, 4, figsize=(12, 6), sharey=True, sharex=True)
axes = axes.ravel()
for i, c in enumerate(SAMPLE_mice):
x = srrs_mn.no_stim[srrs_mn.mouse==c]
y = srrs_mn.log_firingrate[srrs_mn.mouse==c]
# plot obs:
axes[i].scatter(x + np.random.randn(len(x))*0.01, y, alpha=0.4)
# plot both models:
axes[i].plot([0, 1], [unpooled_stim.loc[c, "stim"] , unpooled_no_stim.loc[c, "no_stim"]])
axes[i].plot([0, 1], [a_stim, a_no_stim], "r--")
axes[i].set_xticks([0,1])
axes[i].set_xticklabels(["stim", "no_stim"])
axes[i].set_title(c)
if not i%2:
axes[i].set_ylabel("Log firingrate level")
plt.tight_layout();
<ipython-input-26-757594fd1ada>:25: UserWarning: This figure was using constrained_layout, but that is incompatible with subplots_adjust and/or tight_layout; disabling constrained_layout.
plt.tight_layout();
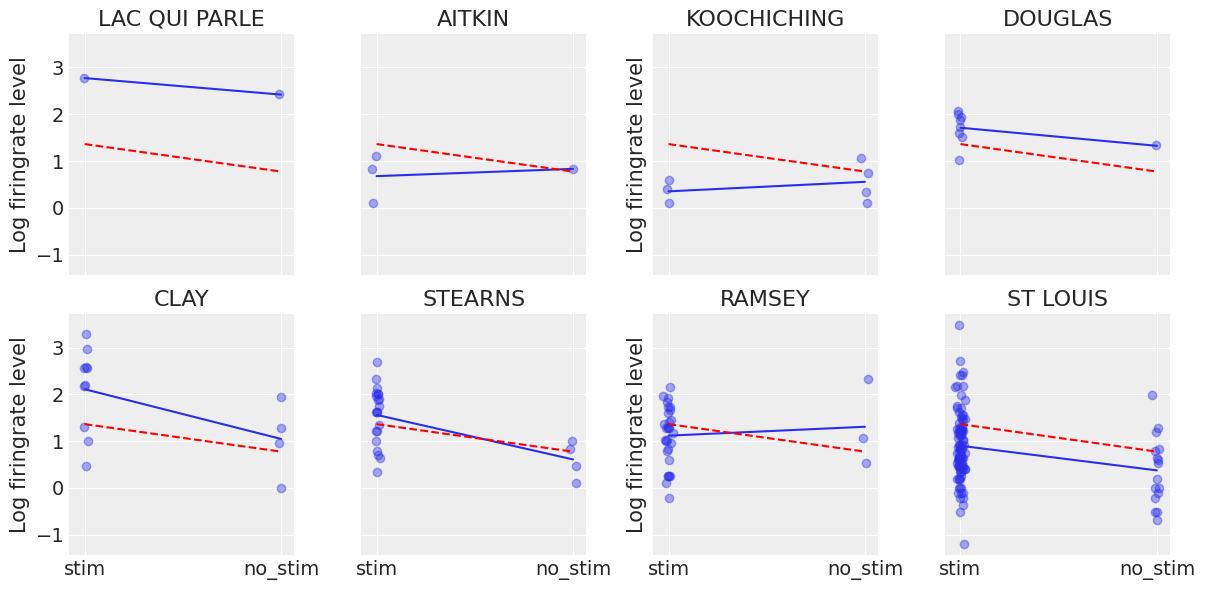
Neither of these models are satisfactory:
If we are trying to identify high-firingrate mice, pooling is useless – because, by definition, the pooled model estimates firingrate at the cohort-level. In other words, pooling leads to maximal underfitting: the variation across mice is not taken into account and only the overall population is estimated.
We do not trust extreme unpooled estimates produced by models using few observations. This leads to maximal overfitting: only the within-mouse variations are taken into account and the overall population (i.e the cohort-level, which tells us about similarites across mice) is not estimated.
This issue is acute for small sample sizes, as seen above: in mice where we have few no_stim measurements, if firingrate levels are higher for those data points than for stim ones (Aitkin, Koochiching, Ramsey), the model will estimate that firingrate levels are higher in no_stims than stims for these mice. But we shouldn’t trust this conclusion, because both scientific knowledge and the situation in other mice tell us that it is usually the reverse (stim firingrate > no_stim firingrate). So unless we have a lot of observations telling us otherwise for a given mouse, we should be skeptical and shrink our mouse-estimates to the cohort-estimates – in other words, we should balance between cluster-level and population-level information, and the amount of shrinkage will depend on how extreme and how numerous the data in each cluster are.
But how do we do that? Well, ladies and gentlemen, let me introduce you to… hierarchical models!
Multilevel and hierarchical models¶
When we pool our data, we imply that they are sampled from the same model. This ignores any variation among sampling units (other than sampling variance) – we assume that mice are all the same:

When we analyze data unpooled, we imply that they are sampled independently from separate models. At the opposite extreme from the pooled case, this approach claims that differences between sampling units are too large to combine them – we assume that mice have no similarity whatsoever:

In a hierarchical model, parameters are viewed as a sample from a population distribution of parameters. Thus, we view them as being neither entirely different or exactly the same. This is partial pooling:

We can use PyMC to easily specify multilevel models, and fit them using Markov chain Monte Carlo.
Partial pooling model¶
The simplest partial pooling model for the neuron firingrate dataset is one which simply estimates firingrate levels, without any predictors at any level. A partial pooling model represents a compromise between the pooled and unpooled extremes, approximately a weighted average (based on sample size) of the unpooled mouse estimates and the pooled estimates.
Estimates for mice with smaller sample sizes will shrink towards the cohort-wide average.
Estimates for mice with larger sample sizes will be closer to the unpooled mouse estimates and will influence the the cohort-wide average.
# HIDE CODE
with pm.Model() as partial_pooling:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=10.)
sigma_a = pm.Exponential('sigma_a', 1.)
# Varying intercepts:
a_mouse = pm.Normal('a_mouse', mu=a, sigma=sigma_a, shape=n_mice)
# Expected value per mouse:
theta = a_mouse[mouse]
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(partial_pooling)
# HIDE CODE
with partial_pooling:
partial_pooling_trace = pm.sample(1000, tune=2000, random_seed=RANDOM_SEED)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, a_mouse, sigma_a, a]
Sampling 4 chains for 2_000 tune and 1_000 draw iterations (8_000 + 4_000 draws total) took 6 seconds.
The number of effective samples is smaller than 25% for some parameters.
To compare partial-pooling and no-pooling estimates, let’s run the unpooled model without the no_stim predictor:
# HIDE CODE
with pm.Model() as unpooled_bis:
a_mouse = pm.Normal('a_mouse', 0., sigma=10., shape=n_mice)
theta = a_mouse[mouse]
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
unpooled_trace_bis = pm.sample(1000, tune=2000, random_seed=RANDOM_SEED)
pm.model_to_graphviz(unpooled_bis)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, a_mouse]
Sampling 4 chains for 2_000 tune and 1_000 draw iterations (8_000 + 4_000 draws total) took 5 seconds.
Now let’s compare both models’ estimates for all 85 mice. We’ll plot the estimates against each mouse’s sample size, to let you see more clearly what hierarchical models bring to the table:
# HIDE CODE
N_mouse = srrs_mn.groupby("mouse")["idnum"].count().values
fig, axes = plt.subplots(1, 2, figsize=(10, 4), sharex=True, sharey=True)
for ax, trace, level in zip(
axes,
[unpooled_trace_bis["a_mouse"], partial_pooling_trace["a_mouse"]],
["no pooling", "partial pooling"]
):
ax.hlines(
partial_pooling_trace["a"].mean(),
0.9,
max(N_mouse) + 1,
alpha=0.4,
ls="--",
label="Est. population mean"
)
for n, l, h in zip(
N_mouse,
az.hdi(trace)[:, 0],
az.hdi(trace)[:, 1]
):
ax.plot([n, n], [l, h], alpha=0.5, c="orange")
ax.scatter(N_mouse, trace.mean(0), alpha=0.8)
ax.set(
title=f"{level.title()} Estimates",
xlabel="Nbr obs in mouse (log scale)",
xscale="log",
ylabel="Log firingrate"
)
ax.legend(fontsize=10)
plt.tight_layout();
<ipython-input-30-898c5d84cc09>:33: UserWarning: This figure was using constrained_layout, but that is incompatible with subplots_adjust and/or tight_layout; disabling constrained_layout.
plt.tight_layout();
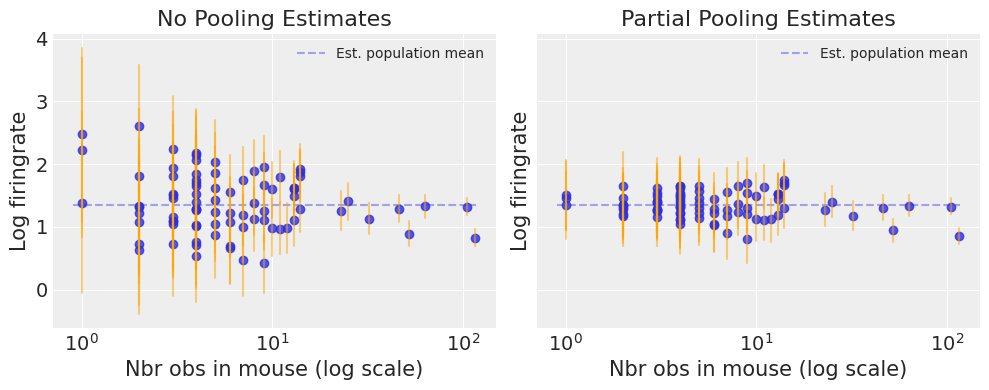
Notice the difference between the unpooled and partially-pooled estimates, particularly at smaller sample sizes: As expected, the former are both more extreme and more imprecise. Indeed, in the partially-pooled model, estimates in small-sample-size mice are informed by the population parameters – hence more precise estimates. Moreover, the smaller the sample size, the more regression towards the overall mean (the dashed gray line) – hence less extreme estimates. In other words, the model is skeptical of extreme deviations from the population mean in mice where data is sparse.
Now let’s try to integrate the no_stim predictor! To show you an example with a slope we’re gonna take the indicator variable road, but we could stay with the index variable approach that we used for the no-pooling model. Then we would have one intercept for each category – stim and no_stim.
Varying intercept model¶
As above, this model allows intercepts to vary across mouse, according to a random effect. We just add a fixed slope for the predictor (i.e all mice will have the same slope):
where
and the intercept random effect:
As with the the no-pooling model, we set a separate intercept for each mouse, but rather than fitting separate regression models for each mouse, multilevel modeling shares strength among mice, allowing for more reasonable inference in mice with little data. Here is what that looks in code:
# HIDE CODE
with pm.Model() as varying_intercept:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=10.)
sigma_a = pm.Exponential('sigma_a', 1.)
# Varying intercepts:
a_mouse = pm.Normal('a_mouse', mu=a, sigma=sigma_a, shape=n_mice)
# Common slope:
b = pm.Normal('b', mu=0., sigma=10.)
# Expected value per mouse:
theta = a_mouse[mouse] + b * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(varying_intercept)
Let’s fit this bad boy with MCMC:
# HIDE CODE
with varying_intercept:
varying_intercept_trace = pm.sample(1000, tune=2000, random_seed=RANDOM_SEED)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, b, a_mouse, sigma_a, a]
Sampling 4 chains for 2_000 tune and 1_000 draw iterations (8_000 + 4_000 draws total) took 6 seconds.
# HIDE CODE
az.plot_forest(varying_intercept_trace, var_names=["a", "a_mouse"], r_hat=True, combined=True);
# HIDE CODE
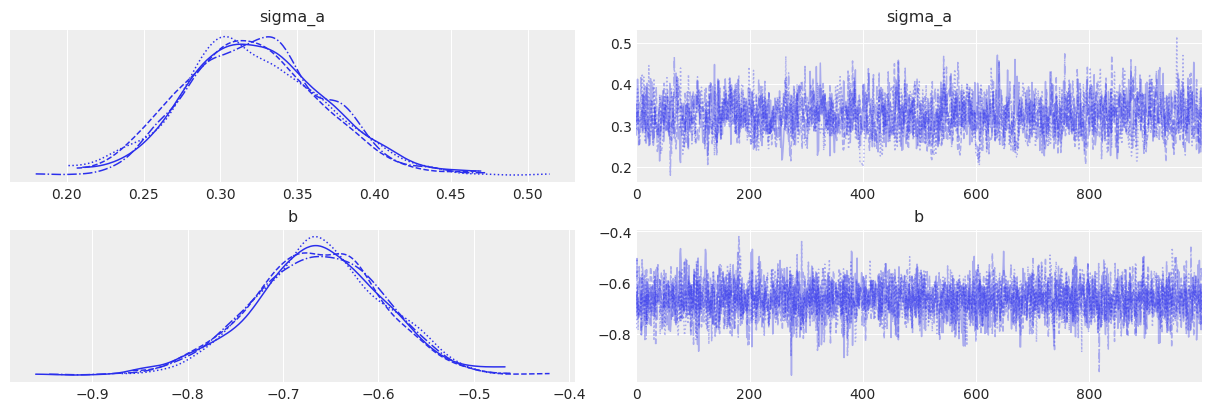
az.plot_trace(varying_intercept_trace, var_names=["sigma_a", "b"]);
# HIDE CODE
az.summary(varying_intercept_trace, var_names=["a", "sigma_a", "b", "sigma"], round_to=2)
| mean | sd | hdi_3% | hdi_97% | mcse_mean | mcse_sd | ess_bulk | ess_tail | r_hat | |
|---|---|---|---|---|---|---|---|---|---|
| a | 1.49 | 0.05 | 1.40 | 1.59 | 0.0 | 0.0 | 2258.17 | 2569.27 | 1.00 |
| sigma_a | 0.32 | 0.04 | 0.24 | 0.41 | 0.0 | 0.0 | 1212.96 | 1615.95 | 1.01 |
| b | -0.66 | 0.07 | -0.80 | -0.54 | 0.0 | 0.0 | 3302.85 | 2909.08 | 1.00 |
| sigma | 0.73 | 0.02 | 0.69 | 0.76 | 0.0 | 0.0 | 5015.66 | 2859.70 | 1.00 |
As we suspected, the estimate for the no_stim coefficient is reliably negative and centered around -0.66. This can be interpreted as neurons without stims having about half (\(\exp(-0.66) = 0.52\)) the firingrate levels of those with stims, after accounting for mouse. Note that this is only the relative effect of no_stim on firingrate levels: conditional on being in a given mouse, firingrate is expected to be half lower in neurons without stims than in neurons with. To see how much difference a stim makes on the absolute level of firingrate, we’d have to push the parameters through the model, as we do with posterior predictive checks and as we’ll do just now.
# HIDE CODE
xvals = np.arange(2)
avg_a_mouse = varying_intercept_trace["a_mouse"].mean(0)
avg_b = varying_intercept_trace["b"].mean()
for a_c in avg_a_mouse:
plt.plot(xvals, a_c + avg_b * xvals, 'ko-', alpha=0.2)
plt.xticks([0,1], ["stim", "no_stim"])
plt.ylabel("Mean log firingrate")
plt.title("MEAN LOG firingrate BY mouse");
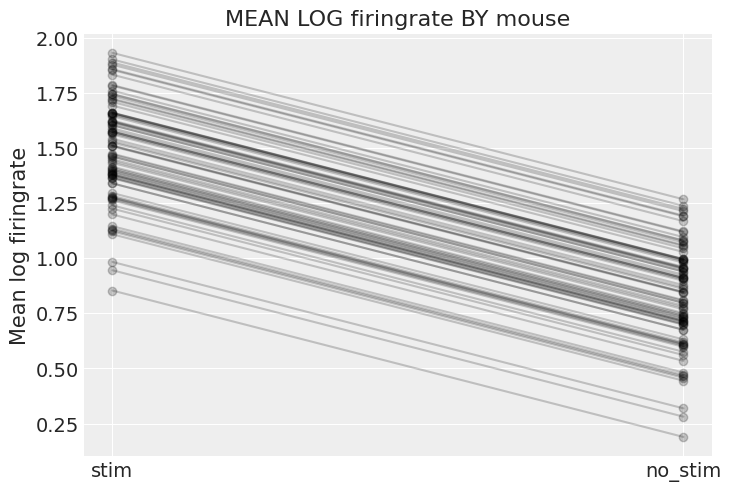
The graph above shows, for each mouse, the expected log firingrate level and the average effect of having no stim – these are the absolute effects we were talking about. Two caveats though:
This graph doesn’t show the uncertainty for each mouse – how confident are we that the average estimates are where the graph shows? For that we’d need to combine the uncertainty in
a_mouseandb, and this would of course vary by mouse. I didn’t show it here because the graph would get cluttered, but go ahead and do it for a subset of mice.These are only average estimates at the mouse-level (
thetain the model): they don’t take into account the variation by neuron. To add this other layer of uncertainty we’d need to take stock of the effect ofsigmaand generate samples from theyvariable to see the effect on given neurons (that’s exactly the role of posterior predictive checks).
That being said, it is easy to show that the partial pooling model provides more objectively reasonable estimates than either the pooled or unpooled models, at least for mice with small sample sizes:
# HIDE CODE
fig, axes = plt.subplots(2, 4, figsize=(15, 7), sharey=True, sharex=True)
axes = axes.ravel()
for i, c in enumerate(SAMPLE_mice):
x = srrs_mn.no_stim[srrs_mn.mouse==c]
y = srrs_mn.log_firingrate[srrs_mn.mouse==c]
# plot obs:
axes[i].scatter(x + np.random.randn(len(x))*0.01, y, alpha=0.4)
# complete-pooling model:
axes[i].plot([0, 1], [a_stim, a_no_stim], "r--", alpha=0.5, label="Complete pooling")
# no-pooling model:
axes[i].plot(
[0, 1],
[unpooled_stim.loc[c, "stim"] , unpooled_no_stim.loc[c, "no_stim"]],
"k:",
alpha=0.5,
label="No pooling"
)
# partial-pooling model:
axes[i].plot(
[0, 1],
[avg_a_mouse[mouse_lookup[c]], avg_a_mouse[mouse_lookup[c]] + avg_b],
label="Partial pooling"
)
axes[i].set_xticks([0,1])
axes[i].set_xticklabels(["stim", "no_stim"])
axes[i].set_title(c)
if not i%2:
axes[i].set_ylabel("Log firingrate level")
if not i%4:
axes[i].legend(fontsize=8, frameon=True)
plt.tight_layout();
<ipython-input-37-42bab39d02e7>:35: UserWarning: This figure was using constrained_layout, but that is incompatible with subplots_adjust and/or tight_layout; disabling constrained_layout.
plt.tight_layout();
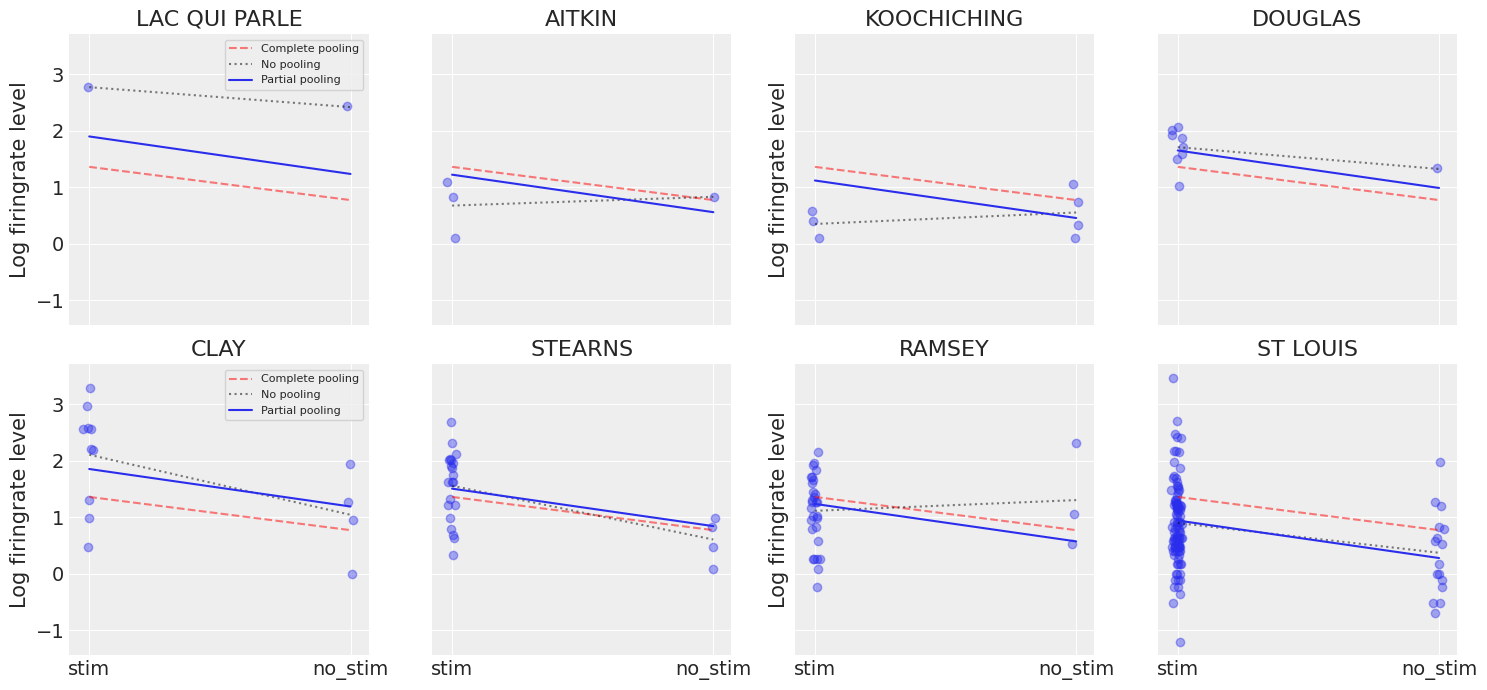
Here we clearly see the notion that partial-pooling is a compromise between no pooling and complete pooling, as its mean estimates are usually between the other models’ estimates. And interestingly, the bigger (smaller) the sample size in a given mouse, the closer the partial-pooling estimates are to the no-pooling (complete-pooling) estimates.
We see however that mice vary by more than just their baseline rates: the effect of no_stim seems to be different from one mouse to another. It would be great if our model could take that into account, wouldn’t it? Well to do that, we need to allow the slope to vary by mouse – not only the intercept – and here is how you can do it with PyMC3.
Varying intercept and slope model¶
The most general model allows both the intercept and slope to vary by mouse:
# HIDE CODE
with pm.Model() as varying_intercept_slope:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=5.)
sigma_a = pm.Exponential('sigma_a', 1.)
b = pm.Normal('b', mu=0., sigma=1.)
sigma_b = pm.Exponential('sigma_b', 0.5)
# Varying intercepts:
a_mouse = pm.Normal('a_mouse', mu=a, sigma=sigma_a, shape=n_mice)
# Varying slopes:
b_mouse = pm.Normal('b_mouse', mu=b, sigma=sigma_b, shape=n_mice)
# Expected value per mouse:
theta = a_mouse[mouse] + b_mouse[mouse] * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(varying_intercept_slope)
Reparametrization¶
Now, if you run this model, you’ll get divergences (some or a lot, depending on your random seed). We don’t want that – divergences are your Voldemort to your models. In these situations it’s usually wise to reparametrize your model using the “non-centered parametrization” (I know, it’s really not a great term, but please indulge me). We’re not gonna explain it here, but there are great resources out there. In a nutshell, it’s an algebraic trick that helps computation but leaves the model unchanged – the model is statistically equivalent to the “centered” version. In that case, here is what it would look like:
# HIDE CODE
with pm.Model() as varying_intercept_slope:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=5.)
sigma_a = pm.Exponential('sigma_a', 1.)
b = pm.Normal('b', mu=0., sigma=1.)
sigma_b = pm.Exponential('sigma_b', 0.5)
# Varying intercepts:
za_mouse = pm.Normal('za_mouse', mu=0., sigma=1., shape=n_mice)
# Varying slopes:
zb_mouse = pm.Normal('zb_mouse', mu=0., sigma=1., shape=n_mice)
# Expected value per mouse:
theta = (a + za_mouse[mouse] * sigma_a) + (b + zb_mouse[mouse] * sigma_b) * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(varying_intercept_slope)
# HIDE CODE
with pm.Model() as varying_intercept_slope:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=5.)
sigma_a = pm.Exponential('sigma_a', 1.)
b = pm.Normal('b', mu=0., sigma=1.)
sigma_b = pm.Exponential('sigma_b', 0.5)
# Varying intercepts:
za_mouse = pm.Normal('za_mouse', mu=0., sigma=1., shape=n_mice)
# Varying slopes:
zb_mouse = pm.Normal('zb_mouse', mu=0., sigma=1., shape=n_mice)
# Expected value per mouse:
aa=pm.Deterministic('aa', a + za_mouse[mouse] * sigma_a)
bb = pm.Deterministic('bb', b + zb_mouse[mouse] * sigma_b)
theta = aa + bb * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(varying_intercept_slope)
# HIDE CODE
with varying_intercept_slope:
varying_intercept_slope_trace = pm.sample(1000, tune=6000, target_accept=0.99, random_seed=RANDOM_SEED)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, zb_mouse, za_mouse, sigma_b, b, sigma_a, a]
Sampling 4 chains for 6_000 tune and 1_000 draw iterations (24_000 + 4_000 draws total) took 89 seconds.
The number of effective samples is smaller than 25% for some parameters.
True, the code is uglier (for you, not for the computer), but:
The interpretation stays pretty much the same:
aandbare still the mean cohort-wide intercept and slope.sigma_aandsigma_bstill estimate the dispersion across mice of the intercepts and slopes (the more alike the mice, the smaller the corresponding sigma). The big change is that now the mice estimates (za_mouseandzb_mouse) are z-scores. But the strategy to see what this means for mean firingrate levels per mouse is the same: push all these parameters through the model to get samples fromtheta.We don’t have any divergence: the model sampled more efficiently and converged more quickly than in the centered form.
Notice however that we had to increase the number of tuning steps. Looking at the trace helps us understand why:
# HIDE CODE
az.plot_trace(varying_intercept_slope_trace, compact=True);
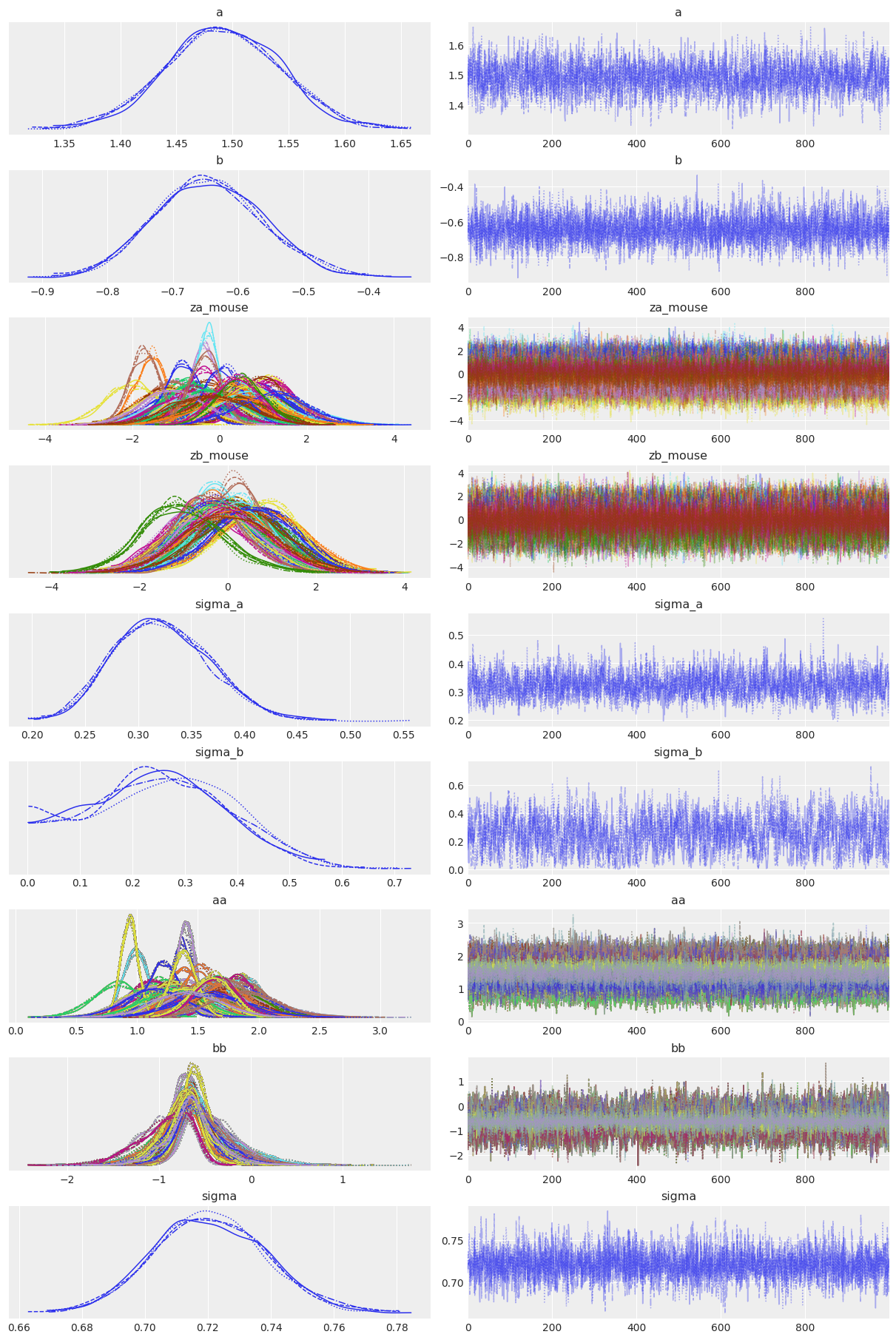
All chains look good and we get a negative b coefficient, illustrating the mean downward effect of no-stim on firingrate levels at the cohort level. But notice that sigma_b often gets very near zero – which would indicate that mice don’t vary that much in their answer to the no_stim “treatment”. That’s probably what bugged MCMC when using the centered parametrization: these situations usually yield a weird geometry for the sampler, causing the divergences. In other words, the non-centered form often perfoms better when one of the sigmas gets close to zero. But here, even with the non-centered model the sampler is not that comfortable with sigma_b: in fact if you look at the estimates with az.summary you’ll probably see that the number of effective samples is quite low for sigma_b.
Also note that sigma_a is not that big either – i.e mice do differ in their baseline firingrate levels, but not by a lot. However we don’t that much of a problem to sample from this distribution because it’s much narrower than sigma_b and doesn’t get dangerously close to 0.
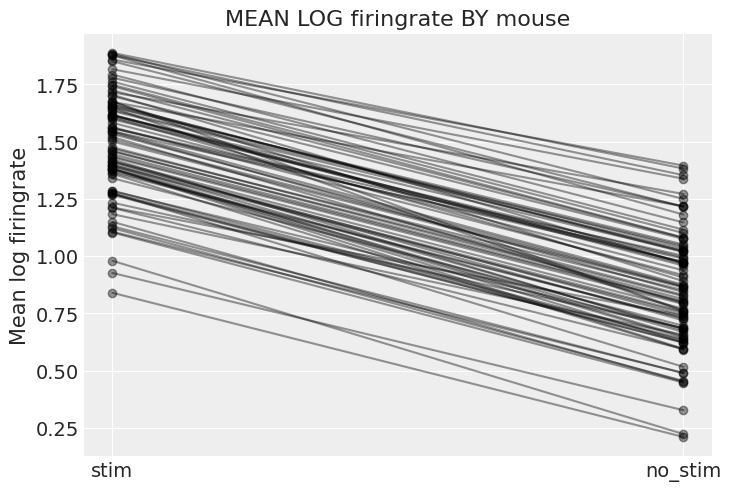
To wrap up this model, let’s plot the relationship between firingrate and no_stim for each mouse:
# HIDE CODE
xvals = np.arange(2)
avg_a_mouse = (
varying_intercept_slope_trace["a"].mean()
+ varying_intercept_slope_trace["za_mouse"].mean(0)
* varying_intercept_slope_trace["sigma_a"].mean()
)
avg_b_mouse = (
varying_intercept_slope_trace["b"].mean()
+ varying_intercept_slope_trace["zb_mouse"].mean(0)
* varying_intercept_slope_trace["sigma_b"].mean()
)
for a_c, b_c in zip(avg_a_mouse, avg_b_mouse):
plt.plot(xvals, a_c + b_c * xvals, 'ko-', alpha=0.4)
plt.xticks([0,1], ["stim", "no_stim"])
plt.ylabel("Mean log firingrate")
plt.title("MEAN LOG firingrate BY mouse");
# HIDE CODE
_=az.plot_posterior(varying_intercept_slope_trace)
/home/m/anaconda3/envs/dj/lib/python3.8/site-packages/arviz/plots/plot_utils.py:490: UserWarning: rcParams['plot.max_subplots'] (40) is smaller than the number of variables to plot (2013) in plot_posterior, generating only 40 plots
warnings.warn(
With the same caveats as earlier, we can see that now both the intercept and the slope vary by mouse – and isn’t that a marvel of statistics? But wait, there is more!
We can (and maybe should) take into account the covariation between intercepts and slopes: when baseline firingrate is low in a given mouse, maybe that means the difference between no_stim and stim measurements will decrease – because there isn’t that much firingrate anyway. That would translate into a positive correlation between a_mouse and b_mouse, and adding that into our model would make even more efficient use the available data.
Or maybe the correlation is negative? In any case, we can’t know that unless we model it. To do that, we’ll use a multivariate Normal distribution instead of two different Normals for a_mouse and b_mouse. This simply means that each mouse’s parameters come from a common distribution with mean a for intercepts and b for slopes, and slopes and intercepts co-vary according to the covariance matrix S. In mathematical form:
where \(\alpha\) and \(\beta\) are the mean intercept and slope respectively, \(\sigma_{\alpha}\) and \(\sigma_{\beta}\) represent the variation in intercepts and slopes respectively, and \(P\) is the correlation matrix of intercepts and slopes. In this case, as their is only one slope, \(P\) contains only one relevant figure: the correlation between \(\alpha\) and \(\beta\).
This translates quite easily in PyMC3:
# HIDE CODE
with pm.Model() as covariation_intercept_slope:
# prior stddev in intercepts & slopes (variation across mice):
sd_dist = pm.Exponential.dist(0.5)
packed_chol = pm.LKJCholeskyCov('packed_chol', eta=2, n=2, sd_dist=sd_dist)
chol = pm.expand_packed_triangular(2, packed_chol, lower=True)
# extract standard deviations and rho:
cov = pm.math.dot(chol, chol.T)
sigma_ab = pm.Deterministic('sigma_ab', tt.sqrt(tt.diag(cov)))
corr = tt.diag(sigma_ab**-1).dot(cov.dot(tt.diag(sigma_ab**-1)))
r = pm.Deterministic('Rho', corr[np.triu_indices(2, k=1)])
# prior for average intercept:
a = pm.Normal('a', mu=0., sigma=5.)
# prior for average slope:
b = pm.Normal('b', mu=0., sigma=1.)
# population of varying effects:
ab_mouse = pm.MvNormal('ab_mouse', mu=tt.stack([a, b]), chol=chol, shape=(n_mice, 2))
# Expected value per mouse:
theta = ab_mouse[mouse, 0] + ab_mouse[mouse, 1] * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(covariation_intercept_slope)
This is by far the most complex model we’ve done so far, so it’s normal if you’re confused. Just take some time to let it sink in. The centered version mirrors the mathematical notions very closely, so you should be able to get the gist of it. Of course, you guessed it, we’re gonna need the non-centered version. There is actually just one change:
# HIDE CODE
with pm.Model() as covariation_intercept_slope:
# prior stddev in intercepts & slopes (variation across mice):
sd_dist = pm.Exponential.dist(0.5)
packed_chol = pm.LKJCholeskyCov('packed_chol', eta=2, n=2, sd_dist=sd_dist)
chol = pm.expand_packed_triangular(2, packed_chol, lower=True)
# extract standard deviations and rho:
cov = pm.math.dot(chol, chol.T)
sigma_ab = pm.Deterministic('sigma_ab', tt.sqrt(tt.diag(cov)))
corr = tt.diag(sigma_ab**-1).dot(cov.dot(tt.diag(sigma_ab**-1)))
r = pm.Deterministic('Rho', corr[np.triu_indices(2, k=1)])
# prior for average intercept:
a = pm.Normal('a', mu=0., sigma=5.)
# prior for average slope:
b = pm.Normal('b', mu=0., sigma=1.)
# population of varying effects:
z = pm.Normal('z', 0., 1., shape=(2, n_mice))
ab_mouse = pm.Deterministic('ab_mouse', tt.dot(chol, z).T)
# Expected value per mouse:
theta = a + ab_mouse[mouse, 0] + (b + ab_mouse[mouse, 1]) * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
covariation_intercept_slope_trace = pm.sample(1000, tune=6000, target_accept=0.99, random_seed=RANDOM_SEED)
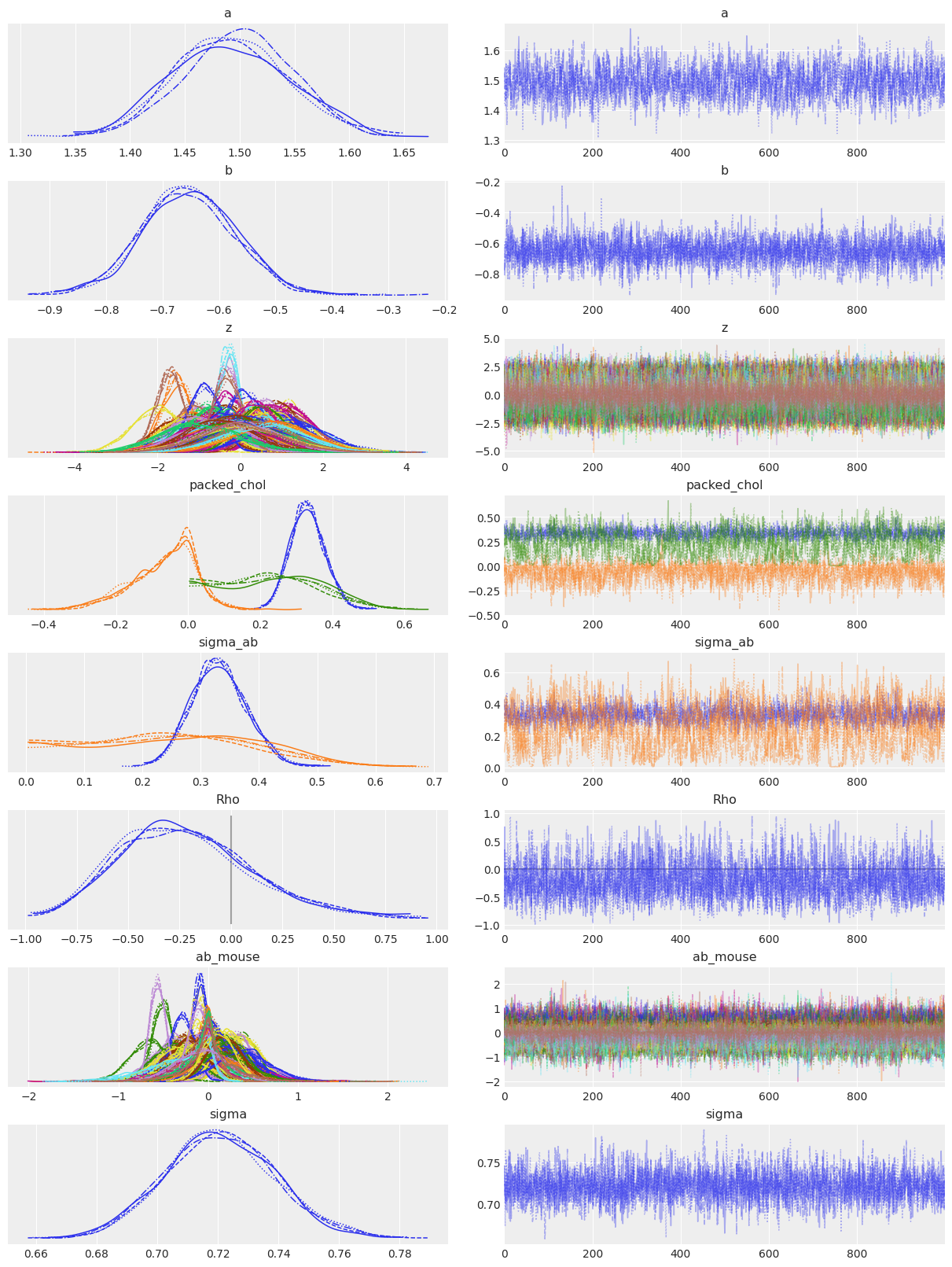
az.plot_trace(covariation_intercept_slope_trace, lines=[("Rho", {}, 0.)], compact=True);
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, z, b, a, packed_chol]
Sampling 4 chains for 6_000 tune and 1_000 draw iterations (24_000 + 4_000 draws total) took 218 seconds.
The number of effective samples is smaller than 25% for some parameters.
# HIDE CODE
pm.model_to_graphviz(covariation_intercept_slope)
So the correlation between slopes and intercepts seems to be negative: when a_mouse increases, b_mouse tends to decrease. In other words, when stim firingrate in a mouse gets bigger, the difference with no_stim firingrate tends to get bigger too (because no_stim readings get smaller while stim readings get bigger). But again, the uncertainty is wide on Rho so it’s possible the correlation goes the other way around or is simply close to zero.
And how much variation is there across mice? It’s not easy to read sigma_ab above, so let’s do a forest plot and compare the estimates with the model that doesn’t include the covariation between slopes and intercepts:
# HIDE CODE
az.plot_forest(
[varying_intercept_slope_trace, covariation_intercept_slope_trace],
model_names=["No covariation.", "With covariation"],
var_names=["a", "b", "sigma_a", "sigma_b", "sigma_ab", "Rho"],
combined=True,
figsize=(8, 4)
);
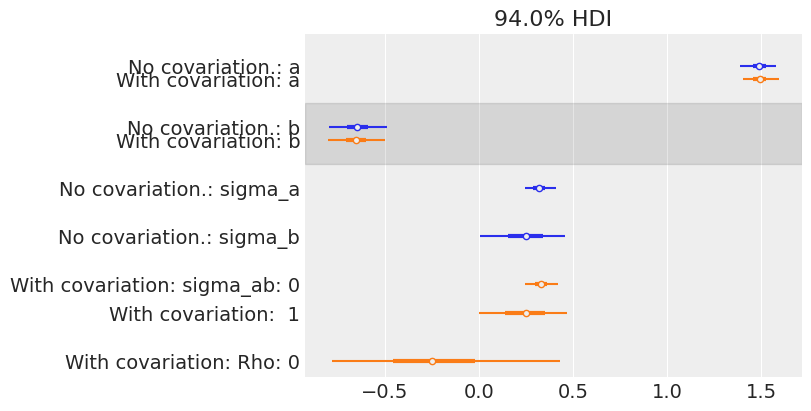
The estimates are very close to each other, both for the means and the standard deviations. But remember, the information given by Rho is only seen at the mouse level: in theory it uses even more information from the data to get an even more informed pooling of information for all mouse parameters. So let’s visually compare estimates of both models at the mouse level:
# HIDE CODE
# posterior means of covariation model:
a_mouse_cov = (
covariation_intercept_slope_trace['a'].mean()
+ covariation_intercept_slope_trace['ab_mouse'].mean(axis=0)[:, 0]
)
b_mouse_cov = (
covariation_intercept_slope_trace['b'].mean()
+ covariation_intercept_slope_trace['ab_mouse'].mean(axis=0)[:, 1]
)
# plot both and connect with lines
plt.scatter(avg_a_mouse, avg_b_mouse, label="No cov estimates", alpha=0.6)
plt.scatter(a_mouse_cov, b_mouse_cov, facecolors='none', edgecolors='k', lw=1, label="With cov estimates", alpha=0.8)
plt.plot([avg_a_mouse, a_mouse_cov], [avg_b_mouse, b_mouse_cov], 'k-', alpha=.5)
plt.xlabel('Intercept')
plt.ylabel('Slope')
plt.legend();
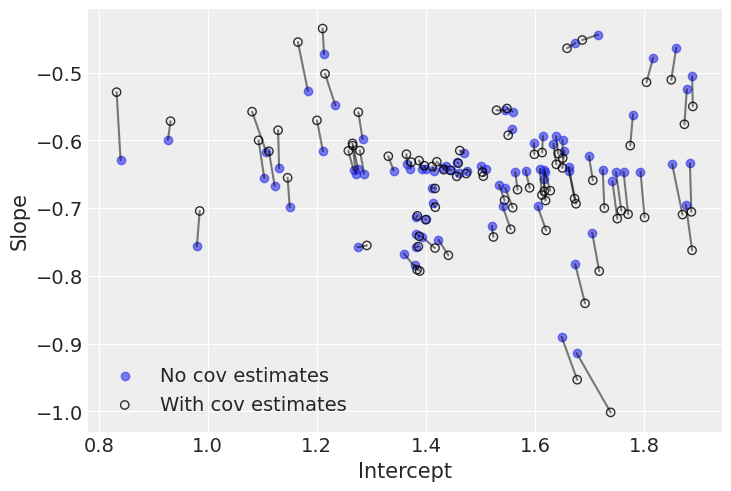
The negative correlation is somewhat clear here: when the intercept increases, the slope decreases. So we understand why the model put most of the posterior weight into negative territory for Rho. Nevertheless, the negativity isn’t that obvious, which is why the model gives a non-trivial posterior probability to the possibility that Rho could in fact be zero or positive.
Interestingly, the differences between both models occur at extreme slope and intercept values. This is because the second model used the slightly negative correlation between intercepts and slopes to adjust their estimates: when intercepts are larger (smaller) than average, the model pushes down (up) the associated slopes.
Globally, there is a lot of agreement here: modeling the correlation didn’t change inference that much. We already saw that firingrate levels tended to be lower in no_stims than stims, and when we checked the posterior distributions of the average effects (a and b) and standard deviations, we noticed that they were almost identical. But on average the model with covariation will be more accurate – because it squeezes additional information from the data, to shrink estimates in both dimensions.
Adding group-level predictors¶
A primary strength of multilevel models is the ability to handle predictors on multiple levels simultaneously. If we consider the varying-intercepts model above:
we may, instead of a simple random effect to describe variation in the expected firingrate value, specify another regression model with a mouse-level covariate. Here, we use the mouse velocity reading \(u_j\) (originally uranium level per county), which is thought to be related to firingrate levels:
Thus, we are now incorporating a neuron-level predictor (no_stim or stim) as well as a mouse-level predictor (velocity).
Note that the model has both indicator variables for each mouse, plus a mouse-level covariate. In classical regression, this would result in collinearity. In a multilevel model, the partial pooling of the intercepts towards the expected value of the group-level linear model avoids this.
Group-level predictors also serve to reduce group-level variation, \(\sigma_{\alpha}\) (here it would be the variation across mice, sigma_a). An important implication of this is that the group-level estimate induces stronger pooling – by definition, a smaller \(\sigma_{\alpha}\) means a stronger shrinkage of mice parameters towards the overall cohort mean.
This is fairly straightforward to implement in PyMC3 – we just add another level:
# HIDE CODE
with pm.Model() as hierarchical_intercept:
# Hyperpriors:
g = pm.Normal('g_neuron', mu=0., sigma=10., shape=2)
sigma_a = pm.Exponential('sigma_a', 1.)
# Varying intercepts velocity model:
a = g[0] + g[1] * u
a_mouse = pm.Normal('a_mouse', mu=a, sigma=sigma_a, shape=n_mice)
# Common slope:
b = pm.Normal('b', mu=0., sigma=1.)
# Expected value per mouse:
theta = a_mouse[mouse] + b * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(hierarchical_intercept)
# HIDE CODE
# compare to no velocity:
with pm.Model() as varying_intercept_slope:
# Hyperpriors:
a = pm.Normal('a', mu=0., sigma=5.)
sigma_a = pm.Exponential('sigma_a', 1.)
b = pm.Normal('b', mu=0., sigma=1.)
sigma_b = pm.Exponential('sigma_b', 0.5)
# Varying intercepts:
a_mouse = pm.Normal('a_mouse', mu=a, sigma=sigma_a, shape=n_mice)
# Varying slopes:
b_mouse = pm.Normal('b_mouse', mu=b, sigma=sigma_b, shape=n_mice)
# Expected value per mouse:
theta = a_mouse[mouse] + b_mouse[mouse] * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(varying_intercept_slope)
Do you see the new level, with sigma_a and g, which is two-dimensional because it contains the linear model for a_mouse? Now, if we run this model we’re gonna get… divergences, you guessed it! So we’re gonna switch to the non-centered form again:
# HIDE CODE
with pm.Model() as hierarchical_intercept:
# Hyperpriors:
g = pm.Normal('g', mu=0., sigma=10., shape=2)
sigma_a = pm.Exponential('sigma_a', 1.)
# Varying intercepts velocity model:
a = pm.Deterministic('a', g[0] + g[1] * u)
za_mouse = pm.Normal('za_mouse', mu=0., sigma=1., shape=n_mice)
a_mouse = pm.Deterministic('a_mouse', a + za_mouse * sigma_a)
# Common slope:
b = pm.Normal('b', mu=0., sigma=1.)
# Expected value per mouse:
theta = a_mouse[mouse] + b * no_stim
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(hierarchical_intercept)
# HIDE CODE
with hierarchical_intercept:
hierarchical_intercept_trace = pm.sample(1000, tune=6000, target_accept=0.99, random_seed=RANDOM_SEED)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, b, za_mouse, sigma_a, g]
Sampling 4 chains for 6_000 tune and 1_000 draw iterations (24_000 + 4_000 draws total) took 50 seconds.
# HIDE CODE
samples = hierarchical_intercept_trace
avg_a = samples['a'].mean(0)
avg_a_mouse = samples['a_mouse'].mean(0)
plt.plot(
u[np.argsort(u)],
avg_a[np.argsort(u)],
"k--",
alpha=0.6,
label="Mean intercept"
)
az.plot_hdi(
u,
samples['a'],
fill_kwargs={"alpha": 0.1, "color": "k", "label": "Mean intercept HPD"}
)
plt.scatter(u, avg_a_mouse, alpha=0.8, label="Mean mouse-intercept")
for ui, l, h in zip(
u,
az.hdi(samples["a_mouse"])[:, 0],
az.hdi(samples["a_mouse"])[:, 1]
):
plt.plot([ui, ui], [l, h], alpha=0.5, c="orange")
plt.xlabel("mouse-level velocity"); plt.ylabel("Intercept estimate")
plt.legend(fontsize=9);
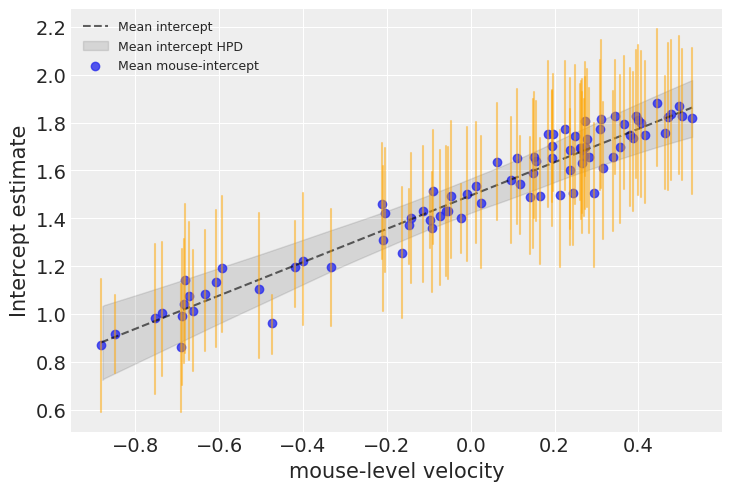
Velocity is indeed much associated with baseline firingrate levels in each mouse. The graph above shows the average relationship and its uncertainty: the baseline firingrate level in an average mouse as a function of velocity, as well as the 94% HPD of this firingrate level (grey line and envelope). The blue points and orange bars represent the relationship between baseline firingrate and velocity, but now for each mouse. As you see, the uncertainty is bigger now, because it adds on top of the average uncertainty – each mouse has its idyosyncracies after all.
If we compare the mouse-intercepts for this model with those of the partial-pooling model without a mouse-level covariate:
# HIDE CODE
az.plot_forest(
[varying_intercept_trace, hierarchical_intercept_trace],
model_names=["W/t. mouse pred.", "With mouse pred."],
var_names=["a_mouse"],
combined=True,
figsize=(6, 40)
);
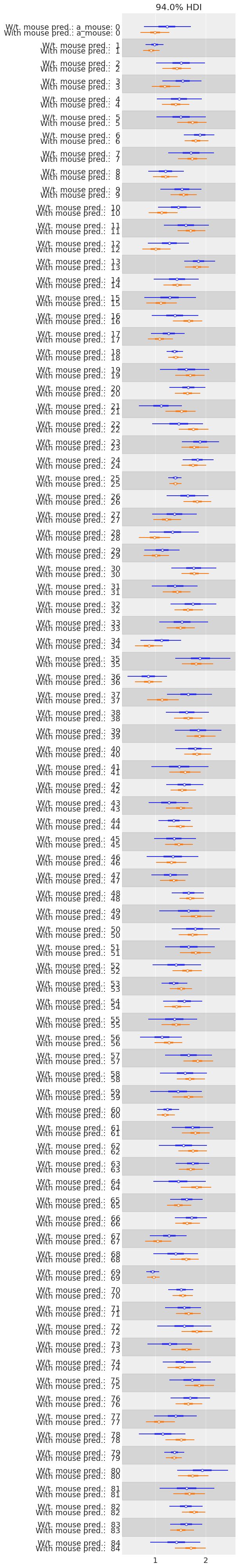
We see that the compatibility intervals are narrower for the model including the mouse-level covariate. This is expected, as the effect of a covariate is to reduce the variation in the outcome variable – provided the covariate is of predictive value. More importantly, with this model we were able to squeeze even more information out of the data.
Correlations among levels¶
In some instances, having predictors at multiple levels can reveal correlation between individual-level variables and group residuals. We can account for this by including the average of the individual predictors as a covariate in the model for the group intercept:
These are broadly referred to as contextual effects.
To add these effects to our model, let’s create a new variable containing the mean of no_stim in each mouse and add that to our previous model:
# HIDE CODE
avg_no_stim = srrs_mn.groupby('mouse')['no_stim'].mean().rename(mouse_lookup).values
with pm.Model() as contextual_effect:
# Hyperpriors:
g = pm.Normal('g', mu=0., sigma=10., shape=3)
sigma_a = pm.Exponential('sigma_a', 1.)
# Varying intercepts velocity model:
a = pm.Deterministic('a', g[0] + g[1] * u + g[2] * avg_no_stim)
za_mouse = pm.Normal('za_mouse', mu=0., sigma=1., shape=n_mice)
a_mouse = pm.Deterministic('a_mouse', a + za_mouse * sigma_a)
# Common slope:
b = pm.Normal('b', mu=0., sigma=1.)
mouse_idx = pm.intX(pm.Data('mouse_idx', mouse))
no_stim_vals = pm.Data('no_stim_vals', no_stim)
# Expected value per mouse:
theta = a_mouse[mouse_idx] + b * no_stim_vals
# Model error:
sigma = pm.Exponential('sigma', 1.)
y = pm.Normal('y', theta, sigma=sigma, observed=log_firingrate)
pm.model_to_graphviz(contextual_effect)
contextual_effect_trace = pm.sample(1000, tune=8000, target_accept=0.99, random_seed=RANDOM_SEED)
az.summary(contextual_effect_trace, var_names=["g"], round_to=2)
Auto-assigning NUTS sampler...
Initializing NUTS using jitter+adapt_diag...
Multiprocess sampling (4 chains in 4 jobs)
NUTS: [sigma, b, za_mouse, sigma_a, g]
Sampling 4 chains for 8_000 tune and 1_000 draw iterations (32_000 + 4_000 draws total) took 76 seconds.
The number of effective samples is smaller than 25% for some parameters.
| mean | sd | hdi_3% | hdi_97% | mcse_mean | mcse_sd | ess_bulk | ess_tail | r_hat | |
|---|---|---|---|---|---|---|---|---|---|
| g[0] | 1.42 | 0.05 | 1.33 | 1.52 | 0.0 | 0.0 | 1663.09 | 2439.39 | 1.0 |
| g[1] | 0.70 | 0.09 | 0.54 | 0.85 | 0.0 | 0.0 | 2532.97 | 3031.95 | 1.0 |
| g[2] | 0.39 | 0.19 | 0.04 | 0.76 | 0.0 | 0.0 | 1861.01 | 2578.32 | 1.0 |
# HIDE CODE
pm.model_to_graphviz(contextual_effect)
So we might infer from this that mice with higher proportions of neurons without stims tend to have higher baseline levels of firingrate. This seems to be new, as up to this point we saw that no_stim was negatively associated with firingrate levels. But remember this was at the neuron-level: firingrate tends to be higher in neurons with stims. But at the mouse-level it seems that the less stims on average in the mouse, the more firingrate. So it’s not that contradictory. What’s more, the estimate for \(\gamma_2\) is quite uncertain and overlaps with zero, so it’s possible that the relationship is not that strong. And finally, let’s note that \(\gamma_2\) estimates something else than velocity’s effect, as this is already taken into account by \(\gamma_1\) – it answers the question “once we know velocity level in the mouse, is there any value in learning about the proportion of neurons without stims?”.
All of this is to say that we shouldn’t interpret this causally: there is no credible mecanism by which a stim (or absence thereof) causes firingrate emissions. More probably, our causal graph is missing something: a confounding variable, one that influences both stim construction and firingrate levels, is lurking somewhere in the dark… Perhaps is it the type of soil, which might influence what type of structures are built and the level of firingrate? Maybe adding this to our model would help with causal inference.
Prediction¶
Gelman (2006) used cross-validation tests to check the prediction error of the unpooled, pooled, and partially-pooled models
root mean squared cross-validation prediction errors:
unpooled = 0.86
pooled = 0.84
multilevel = 0.79
There are two types of prediction that can be made in a multilevel model:
a new individual within an existing group
a new individual within a new group
The first type is the easiest one, as we’ve generally already sampled from the existing group. For this model, the first type of posterior prediction is the only one making sense, as mice are not added or deleted every day. So, if we wanted to make a prediction for, say, a new neuron with no stim in St. Louis mouse, we just need to sample from the firingrate model with the appropriate intercept:
# HIDE CODE
mouse_lookup["ST LOUIS"]
69
That is,
Because we judiciously set the mouse index and no_stim values as shared variables earlier, we can modify them directly to the desired values (69 and 1 respectively) and sample corresponding posterior predictions, without having to redefine and recompile our model. Using the model just above:
# HIDE CODE
with contextual_effect:
pm.set_data({
"mouse_idx": np.array([69]),
"no_stim_vals": np.array([1])
})
stl_pred = pm.sample_posterior_predictive(contextual_effect_trace, random_seed=RANDOM_SEED)
az.plot_posterior(stl_pred);
/home/m/anaconda3/envs/dj/lib/python3.8/site-packages/arviz/data/base.py:169: UserWarning: More chains (4000) than draws (919). Passed array should have shape (chains, draws, *shape)
warnings.warn(
Benefits of Multilevel Models¶
Accounting for natural hierarchical structure of observational data.
Estimation of coefficients for (under-represented) groups.
Incorporating individual- and group-level information when estimating group-level coefficients.
Allowing for variation among individual-level coefficients across groups.
References¶
Gelman, A., & Hill, J. (2006), Data Analysis Using Regression and Multilevel/Hierarchical Models (1st ed.), Cambridge University Press.
Gelman, A. (2006), Multilevel (Hierarchical) modeling: what it can and cannot do, Technometrics, 48(3), 432–435.
McElreath, R. (2020), Statistical Rethinking - A Bayesian Course with Examples in R and Stan (2nd ed.), CRC Press.